BUFFALO, N.Y. — Buffalo has been the only community Tracey Kassman, 65, has ever known. Little did she know, it would be that community that would give her a chance.
In the winter of 2021, she started to notice something was off.
“I started having difficulty in like dropping things,” Kassman said. “I went to my general practitioner in early January, and within a day, I was at Buffalo General.”
Her doctors knew immediately what it was — the deadliest form of brain cancer called glioblastoma, with an average life span of just 12-18 months after diagnosis.
But right when the reality of how little time she had left started to set in, she was given a chance.
“[My doctor] said, ‘But right here at Roswell, we're doing this new study to try to promote a different kind of treatment,’” Kassman said.
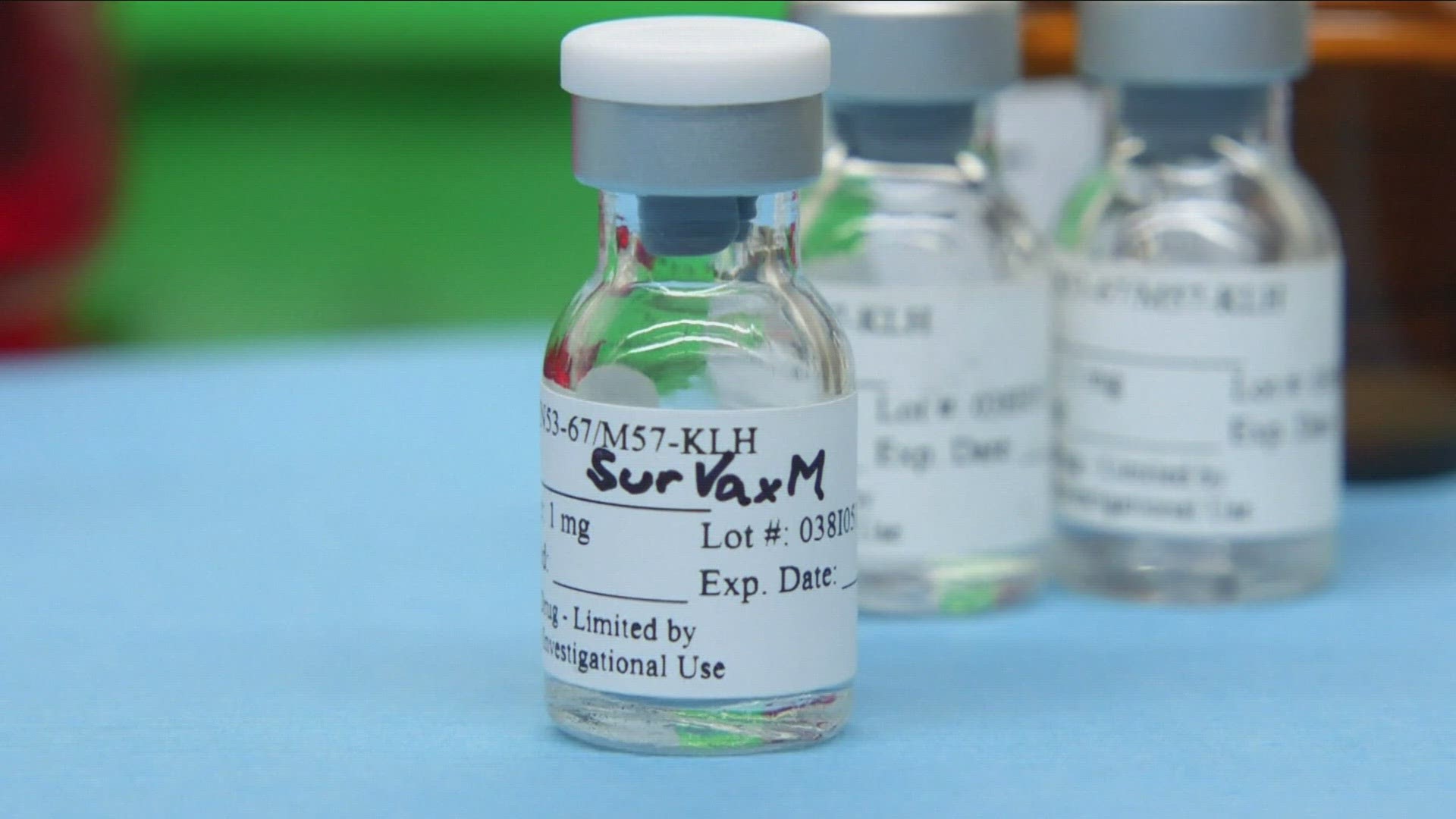
Kassman immediately enrolled in a clinical trial for a new vaccine created at Roswell Park called SurVaxM, which aims to extend the life expectancy of glioblastoma patients.
SurVaxM is an experimental vaccine that targets the protein in tumors called survivin. The idea is that if you get rid of the protein, you get rid of the cancer.
The most recent round of trials saw patients double their life expectancy to on average 26 months after diagnosis.
“The treatment for glioblastoma has not changed in about 20 years,” said Mike Ciesielski, assistant professor of neuro-oncology at Roswell Park and the vaccine’s creator. “The last approved drug for glioblastoma was in 2005, and that's what got us to the point of 15-16 months survival. So this is a milestone in terms of breaking through that wall and potentially getting closer to a cure.”
Ciesielski’s hope is that, once approved, SurVaxM could soon be used to target other cancers, as the vaccine's targeted protein is present in almost 90% of other forms.
“We're making incremental steps,” Ciesielski said. “Any time that a patient gets is really valuable time. It's more birthdays, it's more grandchildren.”
And it’s more time for Kassman, who now a year into treatment, says she feels she’s in the best shape she could be in given her diagnosis.
And while she knows she might not have much time left, she’s thankful she gets to spend even just a little more time with the community that’s always been home.
“It's the world,” she said. “It's everything. Life is about connection, and so the more connection that we can have, the better our life is.”
The vaccine is currently in Phase 2B of clinical trials. In approximately 12-16 months, the doctors will determine whether it is ready to progress to Phase 3 where it will undergo registrational studies to get FDA approval.